Abstract
INTRODUCTION: The availability of imatinib, the first generation tyrosine kinase inhibitor (TKI), has dramatically improved survival of patients with Chronic Myelogenous Leukemia (CML). Second generation TKIs have further advanced patient care. Since loss of exclusivity (LOE) and the entry of a generic imatinib in the US in February 2016, US payers have started realizing savings. However, generic prices have taken two years to decline substantially. Previous work had estimated the anticipated impact of the generic imatinib entry for its first two years, and now the actual prices and savings can be calculated. The objective of this research was to quantify the savings from generic substitution of branded imatinib relative to the potential impact of requiring step therapy through generic imatinib before a 2nd generation TKI.
METHODS: An Excel-based model representing the TKI pharmacy budget of a hypothetical 1-million member commercial plan and 1-million member Medicare plan was developed from a pharmacy only perspective. Historical market share and wholesale acquisition cost pricing over the period of January 2016 to February 2018 were combined with CML age-adjusted US epidemiology data to calculate the monthly spend on TKIs over the same period. Logarithmic regression models were fitted to historical market share data in order to project future utilization of branded TKIs and generic imatinib up to 5 years after LOE. Future drug pricing was assumed to remain constant over the forecasted period. It was assumed that a health plan would not mandate changes in choice of TKI among patients with ongoing TKI treatment. Savings were calculated by comparing the costs of TKIs with generic imatinib to a scenario where generic imatinib was not available. The potential impact of implementing formulary restriction in the TKI class in the next 3 years was explored by applying a step edit to require a trial of generic imatinib before 2nd generation TKIs for incident patients with CML who are new to TKI therapy. Analyses were repeated for the entire commercially and Medicare-covered population in the US based on National Census data
RESULTS: It was estimated that a 1-million member commercial plan saved $0.5M (3%) in the first year and $3.8M (19%) in the second year after LOE. Projected savings significantly increase to $7.8M (37%), $8.2M (39%), and $8.6M (40%) in the 3rd, 4th, and 5th year after LOE, respectively. Initiating a step edit for newly treated CML patients in the commercial plan setting was associated with a very small incremental savings of 1.5% annually over the next 3 years.
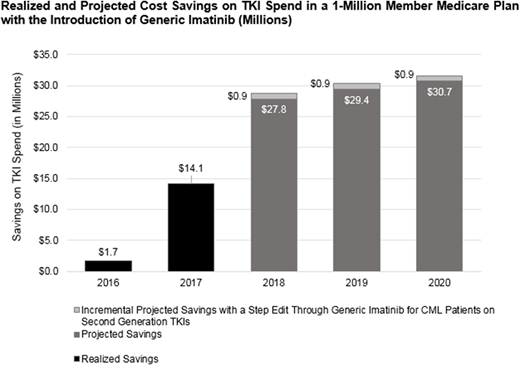
It was estimated that a 1-million member Medicare plan saved $1.7M (3%) and $14M (19%) in the first and second year, respectively. Projected savings in a Medicare plan were $27.8M (37%), $29.4M (39%), and $30.7M (40%), and a step edit for newly treated CML patients gained an additional 1.2% per year in savings over the next 3 years in a Medicare health plan population.
In the first two years, the introduction of generic imatinib saved US payers $1.5B, 7% of the total spend on this drug class. In the next 3 years, the estimated savings are expected to grow to 22% and result in cumulative savings of $8.3B with no management of the class at all. Nearly all of potential savings from the class, 93%, are estimated to come from the generic imatinib conversion from brand with no management required.
CONCLUSIONS: Low generic imatinib price is estimated to result in substantial cost savings to US payers over the next 3 years independent of any formulary management strategies. Formulary management restricting access to 2nd generation TKIs may result in minimal additional savings that are unlikely to reach a threshold of value worthy of action by the health plan.
Bloudek:Xcenda: Employment. Brokars:Bristol-Myers Squibb: Employment. David:Xcenda: Employment. Makenbaeva:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal